In situ Screens hTopo I, IIα (ICE Assays)
Lets face it, sometimes you get misled. There are many promising lead compounds that show promise with purified extracts or enzyme, only to find that your favored drug is unstable or fails to enter the nucleus/cell or for virtually any reason is inactive in the context of a living cell. This is the sort of information you need up front and early on. Rapid decision making is extremely critical. Wouldn’t it make sense to find out if the target (topo) is being hit as an active, endogenous enzyme? Topo enzymes are unusual DNA binding proteins in that they act by making transitory cleavage (or cleavable) complexes with the helix. IFP type drugs (Camptothecin, etoposide) stabilize such complexes and pin the topo down on the template permanently. These DNA/topo complexes are said to be ‘covalent’ since the active site tyrosine hooks up to one or both DNA strands irreversibly leading to a highly stabilized complex that survives harsh treatments like SDS, elevated salt concentration or strong protein denaturants. This makes it possible to quantify such a complex (see In Vivo Link Kit, TG1021,TG1022). In the absence of IFP activity, these refractile complexes are not seen; however, even with low dose, short time (minutes) exposure of a cell to a prototypical IFP drug (VP16), such complexes can be easily detected using the In Vivo Link Kit. A detailed description of the In Vivo Link Kit or ICE Bioassay is available on our YouTube Channel. Using this approach, we can validate the ability of a lead compound to act within the context of a living cell. If this method yields positive data, you can be assured that the mechanism will work in a patient.
- 1 Catalytic Inhibitory Compounds (or CICs) These drugs block or restrict catalytic activity of the enzyme. Typically, CICs will act at steps 1 or 2 in the overall reaction sequence (see Fig. 1). By preventing the non-covalent (electrostatic or ionic bridge) complexes which are necessary to proceed to the cleavage complex (step 3), the reaction is effectively blocked. There are a number of topo II CICs (ICRF-187) and many fewer topo I CICs. Note that CICs can be highly non-specific since even elevated NaCl can be a CIC. In other cases, strong DNA intercalators may interfere with the catalytic reaction sequence. Many in the field simply view a CIC as any agent that can interfere with the ability of a poison (like CPT and topo I) to form cleavage complexes (ie, can ‘reverse or block’ the action of an IFP). This sort of dual drug testing approach can be complicated and we at TopoGEN have the view that it is better to directly test CICs in the absence of other drugs. We can test this within the living cell, by assessing the ability of your test drug to block formation of another IFP control drug (VP16 for topo II or camptothecin for type I mechanisms).
- 2 Interfacial Poisons (or IFPs) These agents are technically poisons because they inhibit the re-ligation step in the sequence (‘poison’ the reaction sequence). Consequently, the enzyme becomes covalently trapped on the DNA target sequence and concurrently DNA cleavage is stabilized (single strand nicked with topo I and double strand DNA break for topo II). Mechanistically, it appears that IFPs intervene stereochemically in the cleavage complex, forming what is called a ´ternary complex´
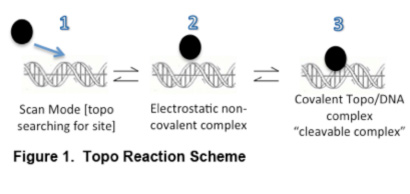 (topo+drug+DNA) wherein the IFP drug induces a mis-alignment of the cleavage intermediate making it unable to re-ligate.When examining events in living cells, the ternary complex is measured using antibodies and genomic DNA, purified by non-protease based method. A Western ‘slot blot’ is used to quantify the topo/genomic DNA complexes, which is a measure of the efficacy of trapping by a given drug
(topo+drug+DNA) wherein the IFP drug induces a mis-alignment of the cleavage intermediate making it unable to re-ligate.When examining events in living cells, the ternary complex is measured using antibodies and genomic DNA, purified by non-protease based method. A Western ‘slot blot’ is used to quantify the topo/genomic DNA complexes, which is a measure of the efficacy of trapping by a given drug
At TopoGEN, we developed, trademarked and patented many of the technologies associated with drug effects on DNA. We are leading experts at drug testing and assessment of drug action from a mechanistic standpoint. When you engage our services, we will describe and lay out a logical/rational approach that is rich in content and strongly mechanistic. We offer independent consultants who can validate our findings. Our services offer expediency and value as well. We can significantly accelerate the pace of your research, produce publication quality data and you will own the intellectual property that comes with our studies. We routinely enter into confidentiality agreements on projects, so your results are protected. Our in-house testing program is flexible and efficient. The following are important points associated with our services:
- 1 All positive and negative controls We demonstrate internally that all testing platforms are perfectly functional. As a result, there is no guessing. Your data will be clear and unambiguous. We stand behind our findings and certify the data.
- 2 Testing Flexibility. We tailor each contract to offer maximum flexibility and coordinate with you over the course of the project to ensure intellectual flow of ideas and results. We will advise you on the best course of action with all hits. You can select the level of detail for the project that best suits your needs.
- 3 Over-run Flexibility. In most if not all projects, there will be a need to re-examine, re-test or alter the course. How do we correct for work-load adjustments in the mid-stream? Our solution: we include on all projects, a small indirect “over-run fee” that will allow us to perform additional key experiments without asking for additional funds. This is an advantage for clients and ensures that our high standards of data quality will be maintained. For example, if additional testing regimens are required, we carry out the service automatically. Importantly, there is no guessing on the budget and you will not pay additional fees.
- 4 Consulting. We are a team of topo experts. All projects include email and direct SKYPE support over the course of the project. Our scientific staff will discuss and suggest future prospects or experiments.
- 5 Reporting. For complete flexibility and to ensure cost-effective contracts, we offer two levels of reporting. At a basic level, included in all contracts, we provide well-documented data (powerpoint, PDF or password protected cloud account). We draw conclusions, presented as bullets, on each slide and make a recommendation for further development and maturation of the project. For clients who wish to have a ‘manuscript’ style report, we offer fully referenced publication style reports. These reports are professionally prepared and are suitable for presentation to regulatory agencies. These publication style reports can be reviewed independently by third party opinion leaders in the field and the reviews appended to the final report.
- 6 Hands-on Experience. The company has been actively engaged in contract drug testing since the 1990s and we have the experience to get results that will enable ‘go/no go’ decision making on drug development.
- 7 Expedience. In most cases, the project will be completed over a period of several business days. We will clearly stipulate how long the project will take and we deliver on time.
- 8 A Selection of Enzymes Target Any topoisomerase that has an associated mono-specific antibody can be evaluated. These include human topo I, topo II, topo II and topo III. We are pioneers in these sorts of targeting studies and developed the methods used by many well established labs in the field.
