Recombinant Human Topo I (Tyrosine Mutant)
This is the catalytically inactive form of mutant human topoisomerase I purified to homogeneity. The mutation is a single residue change at the active site tyrosine (which has been changed to phenylalanine). This preparation is overexpressed in baculovirus and affinity as a single band on SDS-PAGE of 100 kDa (see SDS-PAGE data below). The enzyme cannot relax DNA (see data below) and contains a short (6 residue) histidine tag.
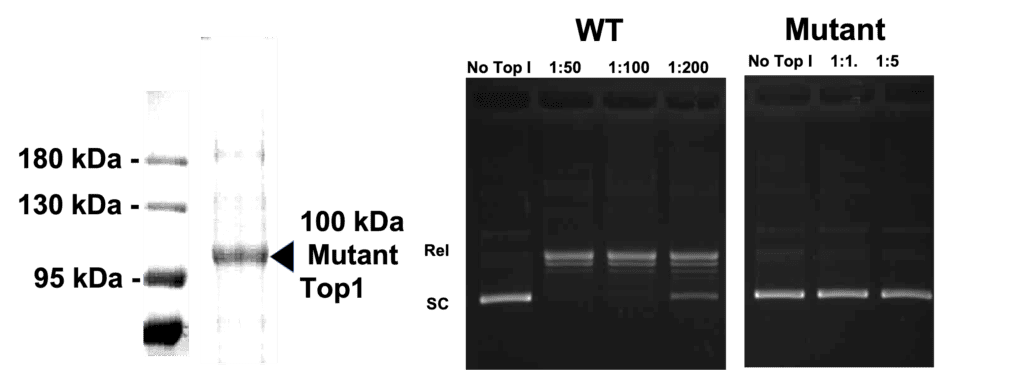
Figure shows an SDS-PAGE of purified mutant Top1. Agarose gel data clearly show the mutant is inactive compared to the Wild Type (WT protein).
Product Contents
-10ug Mutant Human Topoisomerase I Enzyme
-0.5ml 10x Topo I Reaction Buffer
Tyrosine Mutant Human Topo I Quality Control Tests
Relaxation assays were carried out in a final volume of 25 μl in topo I reaction buffer (10X reaction buffer, supplied with this product is: 100 mM Tris-Cl, pH 7.9, 1.5 M NaCl, 1% BSA, 1 mM Spermidine, 50% glycerol). Supercoiled plasmid DNA was included at 0.25 μg/reaction. Reactions terminated with 5 μl (per 20 μl reaction volume) of stop buffer (5% sarkosyl, 0.0025% bromophenol blue, 25% glycerol). Reaction products were analyzed on a 1% native agarose gel. Under these conditions, relaxation activity was not detectable with up to 0.5 ug of mutant protein.
A test for nuclease contamination was carried out by assaying for the formation of linear KDNA and linear plasmid DNA. Incubations of 1 μg of catenated kDNA or supercoiled pUC19 DNA (4 hrs. at 37° C in the presence of 10 mM MgCl2) were performed. Linear DNA or breakdown products were not generated under these conditions.
A check for cross contamination with topo II was negative. There was no decatenation of KDNA in topo II reaction conditions.
The final fraction of mutant topo I is a column pool and is in the following buffer: 20mM NaH2PO4 (pH7.4), 300mM NaCl, 500mM Imidazole. The final fraction was analyzed by SDS-PAGE and shown to contain a single, predominant band of 100 kDa.
The enzyme is shipped on blue ice or dry ice internationally. Store the enzyme at -20°C.


