In situ Screen (cell context) Screening
TopoGEN offers powerful and tractable services to quantify the ability of your front line anticancer drug to specifically target endogenous topoisomerase action in cells.
Lets face it, sometimes you get misled. Many promising lead compounds that appear to act on topo I or II with purified extracts or enzyme, never see the light of day; often because they fail to enter the nucleus/cell, become degraded or interconverted and simply do not hit the target topo for any number of reasons. This is the sort of information you need up front and early on. Rapid decision making is extremely critical. Wouldn’t it make sense to find out if the target (topo) is being hit as an active, endogenous enzyme? Topo enzymes are unusual DNA binding proteins in that they act by making transitory cleavage (or cleavable) complexes with the helix. IFP type drugs (Camptothecin, Etoposide) stabilize such complexes and pin the topo down on the template permanently. These DNA/topo complexes are said to be ‘covalent’ since the active site tyrosine hooks up to one or both DNA strands irreversibly leading to a highly stabilized complex that survives harsh treatments like SDS, elevated salt concentration or strong protein denaturants. This makes it possible to quantify such a complex using either a CsCl step gradient or our newer kit that avoids the density gradient step (TG1020- kits). The latter Kit is a series with different antibodies to interrogate different topoisomerases. Both Kits are based on the same basic concept: In the absence of IFP activity, the covalent complexes are not seen; however, even with low dose, short time (minutes) exposure of a cell to a prototypical IFP drug (VP16), such complexes can be easily detected using these Kits. A detailed description of the In Vivo Link Kit or ICE Bioassay is available on our YouTube Channel. Using this approach, we can validate the ability of a lead compound to act within the context of a living cell. If this method yields positive data, you can be assured that the mechanism will work in a patient.
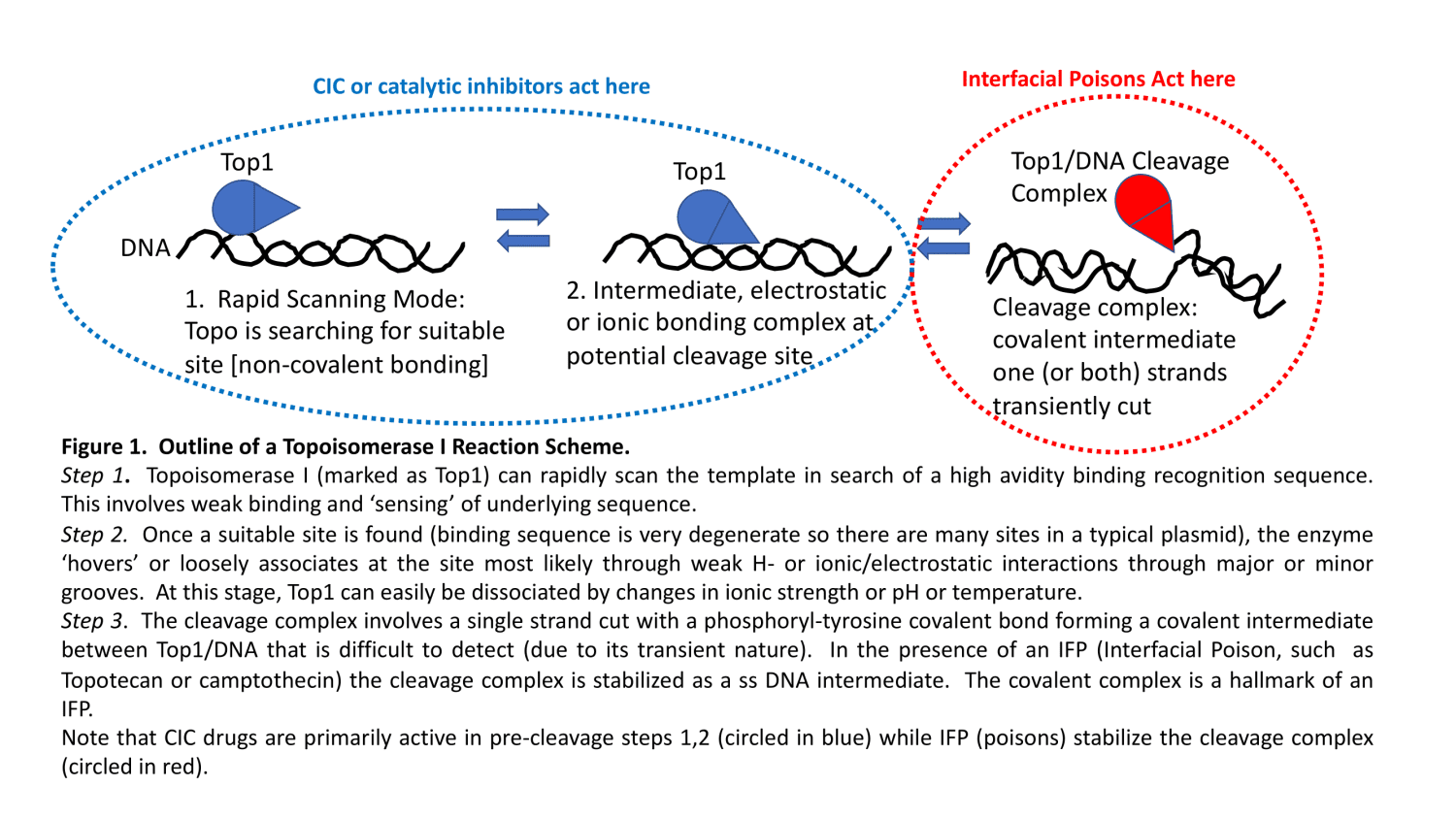
These drugs block or restrict catalytic activity of the enzyme. Typically, CICs will act at steps 1 or 2 in the overall reaction sequence (see Fig. 1). By preventing the non-covalent (electrostatic or ionic bridge) complexes which are necessary to proceed to the cleavage complex (step 3), the reaction is effectively blocked. There are a number of topo II CICs (ICRF-187) and many fewer topo I CICs. Note that CICs can be highly non-specific since even elevated NaCl can be a CIC. In other cases, strong DNA intercalators may interfere with the catalytic reaction sequence. Many in the field simply view a CIC as any agent that can interfere with the ability of a poison (like CPT and topo I) to form cleavage complexes (ie, can ‘reverse or block’ the action of an IFP). This sort of dual drug testing approach can be complicated and we at TopoGEN have the view that it is better to directly test CICs in the absence of other drugs. We can test this within the living cell, by assessing the ability of your test drug to block formation of another IFP control drug (VP16 for topo II or camptothecin for type I mechanisms).
These drugs block or restrict catalytic activity of the enzyme. Typically, CICs will act at steps 1 or 2 in the overall reaction sequence (see Fig. 1). By preventing the non-covalent (electrostatic or ionic bridge) complexes which are necessary to proceed to the cleavage complex (step 3), the reaction is effectively blocked. There are a number of topo II CICs (ICRF-187) and many fewer topo I CICs. Note that CICs can be highly non-specific since even elevated NaCl can be a CIC. In other cases, strong DNA intercalators may interfere with the catalytic reaction sequence. Many in the field simply view a CIC as any agent that can interfere with the ability of a poison (like CPT and topo I) to form cleavage complexes (ie, can ‘reverse or block’ the action of an IFP). This sort of dual drug testing approach can be complicated and we at TopoGEN have the view that it is better to directly test CICs in the absence of other drugs. We can test this within the living cell, by assessing the ability of your test drug to block formation of another IFP control drug (VP16 for topo II or camptothecin for type I mechanisms).
Our Screening Services: Independent Validation, Expediency, Value
At TopoGEN, we developed, trademarked and patented many of the technologies associated with drug effects on DNA. We are leading experts at drug testing and assessment of drug action from a mechanistic standpoint. When you engage our services, we will describe and lay out a logical/rational approach that is rich in content and strongly mechanistic. We offer independent consultants who can validate our findings. Our services offer expediency and value as well. We can significantly accelerate the pace of your research, produce publication quality data and you will own the intellectual property that comes with our studies. We routinely enter into confidentiality agreements on projects, so your results are protected. Our in-house testing program is flexible and efficient. The following are the “Top 10” reasons you should consider our screening services for your R&D needs:
All Postitive & Negative Controls
We demonstrate internally that all testing platforms are perfectly functional. As a result, there is no guessing. Your data will be clear and unambiguous. We stand behind our findings and certify the data.
All Markers will be included
To document and validate the results. Again, we certify tractable results that will be internally controlled.
Testing & Costing Flexibility
We tailor each contract to offer maximum flexibility and coordinate with you over the course of the project to ensure intellectual flow of ideas and results. We will advise you on the best course of action with all hits. You can select the level of detail for the project that best suits your needs. In some projects, there will be a need to re-examine, re-test or alter the course. How do we correct for work-load adjustments in the mid-stream? Our solution: we include on all projects, a small indirect cost that will allow us to perform additional key experiments without asking for additional funds. This is an advantage for clients and ensures that our high standards of data quality will be maintained. For example, if additional testing regimens are required, we carry out the service automatically. Importantly, there is no guessing on the budget and you will not pay additional fees.
Consulting
We are a team of topo experts. All projects include email and direct SKYPE support over the course of the project. Our scientific staff will discuss and suggest future prospects or experiments.
Reporting
For complete flexibility and to ensure cost-effective contracts, we offer two levels of reporting. At a basic level, included in all contracts, we provide well-documented data (PowerPoint, PDF or password protected cloud account). We draw conclusions, presented as bullets, on each slide and make a recommendation for further development and maturation of the project. For clients who wish to have a ‘manuscript’ style report, we offer fully referenced publication style reports. These reports are professionally prepared and are suitable for presentation to regulatory agencies. These publication style reports can be reviewed independently by third party opinion leaders in the field and the reviews appended to the final report.
Hands-on Experience
The company has been actively engaged in contract drug testing since the 1990s and we have the experience to get results that will enable ‘go/no go’ decision making on drug development.
Expedience
In most cases, the project will be completed over a period of several business days. We will clearly stipulate how long the project will take and we deliver on time.
A Selection of Enzymes Target
We can test prokaryotic and eukaryotic systems with highly purified enzymes that are well-controlled reagents. We can provide additional documentation of purity and QC on all preparations. The following enzymes or tractable antibodies are currently in our inventory:
- Human DNA Topoisomerase I
- Human DNA Topoisomerase IIα
- E. coli DNA Gyrase
- S. aureus DNA Gyrase
- E. coli Topoisomerase IV
- S. aureus Topoisomerase IV
Advanced Testing in Living Cells
TopoGEN is a pioneer in testing agents that operate in the context of the cell. For eukaryotic systems, we can perform rigorous testing in tissue culture cells. We offer a variety of cell lines suitable for topo I, IIα and IIβ testing. For example, we can test your drug (either as CIC or IFP) in virtually any cell line of interest.
The "Dual Gel Analytical System
TopoGEN is unique in offering this service as part of our routine testing regimen. In all projects, we design our experiments to garner new information about your compound. For example, all gels are run in duplicate. One is a non-EB gel system, used to demonstrate unambiguous catalytic activity and the other gel is an EB containing gel to parse clearly the cleavage products. Since the reactions come from the same tube, we can be sure that the results are reproducible and accurate. Importantly, the non-EB gel system also reports whether the drug is a DNA intercalator, which may suggest genotoxicity in cells.
Sample data for our ice kit (Cat #TG1020)
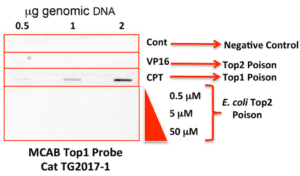
The sample data for our ICE Kit are shown below (Fig. 2,3). The purpose of this sort of analysis is two fold: first, we wish to test whether an unknown drug acts as a topoisomerase poison in the context of a living cell; second, we wish to know whether the unknown is targeting topoisomerase I or topoisomerase IIa (Top1 or Top2a). Because the ICE assay is antibody based, we can easily distinguish between Top1, Top2a, Top2b or even TopIII (all of these antibodies are available through TopoGEN). Basically, we supply reagents and specific antibodies and a detailed protocol with all required reagents. After drug or no drug treatment, cells are lysed in a special lysis buffer that retains Top/DNA covalent adducts, genomic DNA is prepared and fixed amounts of this DNA is placed onto a Western slot blot and probed with anti-Topoisomerase AB of a specific type (provided in the kit). Topo/DNA adducts will co-purify with genomic DNA which are then detected by Western blotting as shown in Figures 2,3 below.
Figure 2 shows a typical result with a Top2 poison like etoposide or VP16. In the absence of VP16, background levels of Top2 are seen due to carryover. More importantly, a substantial increase in signal is detected in the presence of graded amounts of genomic DNA on the blot. Figure 3 demonstrates the specificity of the ICE ASSAY. Cells treated with camptothecin give a potent signal on the blot, whereas cells treated with no drug (negative control) or with VP16 (a Top2 poison) or with a DNA gyrase poison, yielded only background signals (blot probed with an anti-Top1 monoclonal antibody). The data and Figure 3 clearly demonstrates that ONLY Top1 is detected in camptothecin treated cells and attests to the specificity of the ICE Assay Kit.
Fig 2. Representative ICE Blot testing for Top2a. HeLa cells were treated with 50uM VP-16 for 30’ and lysed in Buffer A. DNA from negative controls (no drug) and from drug treated cells was isolated and quantified on a NanoDrop. Indicated amounts of DNA were probed with TopoGEN polyclonal Top2a AB (TG2011-1) The signals in the VP16 slots should be significantly higher than controls a shown. The signal ratio between the +DRUG:No drug control is called the “ICE DELTA” (or ICEΔ). The ICEΔ should be 2-6 fold or more for a valid result.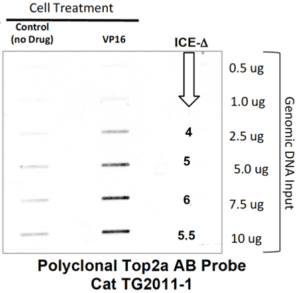
Figure 3. ICE Blot testing for Top1. HeLa cells were untreated (control) or treated with 50uM VP-16 or 50uM Camptothecin for 30’ while in exponential growth and lysed in Buffer A. DNA from negative controls (no drug) and from drug treated cells was isolated and quantified on a NanoDrop. Indicated amounts of DNA were probed with TopoGEN polyclonal Top1 Monoclonal (Cat TG2017-1). To show specificity HeLa cells were treated with an E. coli Top2 poison at three different concentrations shown (30 min at 37oC and lysed with Buffer A). Indicated amounts of DNA (0.5 to 2ug) were slot blotted.